Which Is the Best Description of a Carbonyl Group
Which of the following is a false statement concerning amino groups. The carbon and oxygen in the carbonyl group are sp2-hybridized with bond angles of 120.
Carbonyl Group Definition And Quiz Biology Dictionary
C O sp2 C O the carbonyl group C.
. In general aldehydes are more electrophilic than ketones c. A carbonyl group is a group of atoms that consists of a carbon atom covalently attached to an oxygen atom by a double bond. However the term Carbonyl can also refer to carbon monoxide as the ligand within an organometallic or inorganic compound say a metal carbonyl such as nickel carbonyl.
Carbon 2 in D-glucose is tetrahedral carbon 2 in the conjugate base is trigonal planar D-glucose is uncharged the conjugate base would have a negative charge D-glucose is. O A carbonyl group is a carbon atom double bonded to an oxygen atom and flanked by two alkyl O substituents A carbonyl group is a carbon atom double bonded to an oxygen atom. Which is the best description of a carbonyl group.
In ketones both available bonds on the carbonyl carbon atom are carbon-to-carbon bonds. In the field of organic chemistry a carbonyl group consists of carbon atoms double bonded with some oxygen atoms. An oxygen joined to a carbon by a single covalent bond c.
This preview shows page 1 - 2 out of 2 pages. The difference between the electronegativities of carbon and oxygen is large enough to make the CO bond moderately polar. A nitrogen and two hydrogens joined to a carbon by covalent bonds b.
A carbonyl group consists of carbon and oxygen joined together by a double bond. Which is the best description of a carbonyl group. B a nitrogen and two hydrogens joined to a carbon by covalent bonds.
The carbon atom to satisfy its valence of 4 must also be attached by covalent bonds to two other atoms. Up to 24 cash back Which is the best description of a carbonyl group Cyclopentadienyliron dicarbonyl dimer Names IUPAC name Di-μ-carbonyldicarbonylbisη5-cyclopenta-24-dien-1-yldiiron Other names Biscyclopentadienyltetracarbonyl-diiron Dicyclopentadienyltetracarbonyl-diiron Bisdicarbonylcyclopentadienyliron Identifiers CAS. Question 3 1 point Select all differences between D-glucose and the conjugate base that forms after removal of the proton alpha to the carbonyl group by a strong base.
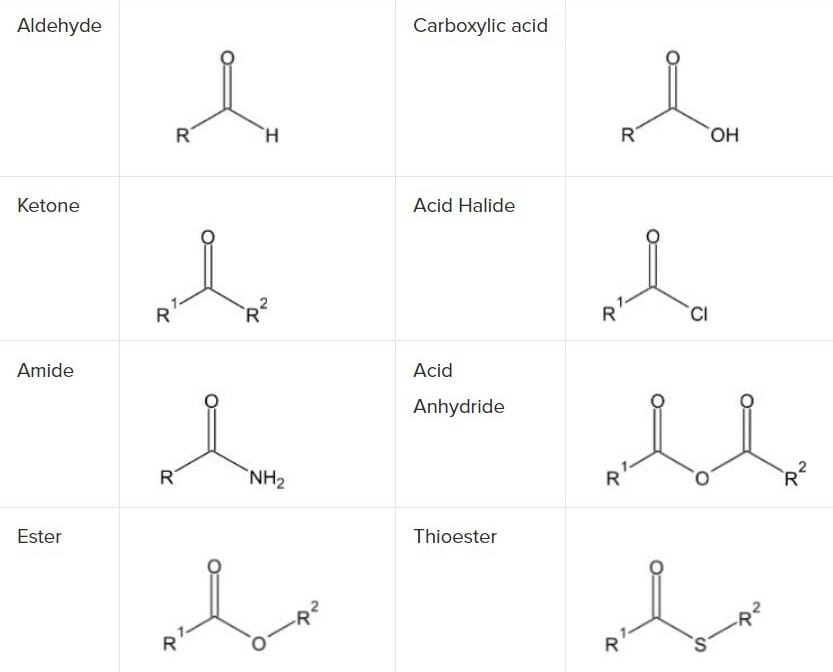
Different enantiomers may have different or opposite physiological effects. However Carbonyl Esters consists of carbonyl group influenced by different alkyl. The joining of carbonyl carbon is with hydrogen on one side in aldehydes whereas the joining of two carbon atoms on both the side of carbonyl carbon in the case of ketones.
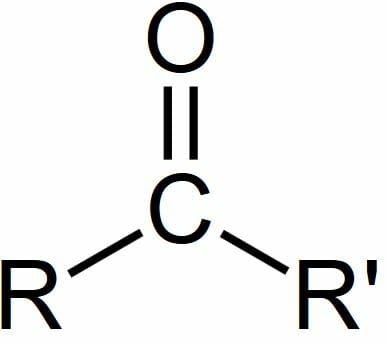
A carbon atom joined to an oxygen by a double covalent bond. The carbon atom of a carbonyl group is electrophilic b. A compound containing a carbonyl group is often referred to as a carbonyl compound.
A carbonyl group is a functional group featuring a double bond between a carbon atom and an oxygen atom illustrated below. It is common to several classes of organic compounds as part of many larger functional groups. When placed in water it.
14 Which is the best description of a carbonyl group. Which of the following contains nitrogen in addition to carbon oxygen and hydrogen. Amino acids consist of an α carbon atom that is bonded to the amine group hydrogen atom carboxylic group and a characteristic R group.
The Carbonyl Group The carbonyl group CO is found in aldehydes ketones and many other organic functional groups. Which is the best description of a carbonyl group. The carbonyl group a carbon-to-oxygen double bond is the defining feature of aldehydes and ketones.
Aldehydes are synthesized by the oxidation of primary alcohols. A a nitrogen and two hydrogens joined to a carbon by covalent bonds B a carbon atom joined to an oxygen by a double covalent bond. O A carbonyl group is a substituent that contains one less hydrogen than the corresponding alkane.
A compound containing a carbonyl group CO In organic chemistry a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom. This structure creates a resonance hybrid structure within the molecule in which the electrons are continuously redistributed between the carbons and the oxygen which can allow the molecule to hold more electrons and. A carbon skeleton is covalently bonded to both an amino group and a carboxyl group.
It is somewhat misleading to write the carbonyl group as a covalent CO double bond. A They are basic in pH. A carbon joined to a hydroxyl group by a single covalent bond d.
The simplest type of molecule that contains a carbonyl group is a ketone. A carbon atom joined to an oxygen by a double covalent bond. Addition of a nucleophile to a carbonyl group changes the hybridization of the carbonyl carbon from sp3 to sp2 hybridization.
What is the best description of a carbonyl group. Nucleophilic addition to carbonyl groups can be catalyzed by acid or base d. In aldehydes at least one bond on the carbonyl group is a carbon-to-hydrogen bond.
Description of the Carbonyl Group. B They are found in amino acids. An amino acid such as glycine.
C a carbon joined to two hydrogens by single covalent bonds. A an oxygen joined to a carbon by a single covalent bond. A carbonyl group is a functional group in biochemistry characterized by a carbon atom double bonded to an oxygen with a larger molecule.
E a carbon atom joined to an oxygen by a double covalent bond. The presence of carboxylic acid group in the amino acids is responsible for the dissociation of protons by the carboxylic acid group that causes the amino acids to behave as an acid. In ketones two carbon groups are attached to the carbonyl carbon while in aldehydes at least one hydrogen is attached to the carbon.
A a nitrogen and two hydrogens joined to a carbon by covalent bonds B a carbon atom joined to an oxygen by a double covalent bond C a sulfur and a hydrogen joined to a carbon by covalent bonds D an oxygen joined to a carbon by a single covalent bond. In organic chemistry a carbonyl group is a functional group where a carbon atom is double bonded to an oxygen atom. 14 Which is the best description of a carbonyl group.
As a result the carbonyl group is best described as a hybrid of the following resonance structures. A great way to remember that youre dealing with carbonyl is to look at the.
Carbonyl Compounds Nomenclature Nucleophilic Addition And More Concise Medical Knowledge

Carbonyl Compounds Carbonyl Group Definition Properties Reactions Applications Of Carbonyl Compounds

0 Response to "Which Is the Best Description of a Carbonyl Group"
Post a Comment